Okay, so today I tried tackling this ATP formula thing in my workbook – you know, Adenosine Triphosphate? Felt like brushing up on basic cell energy stuff. Figured it’d be simple, but man, I stumbled hard into some classic traps. Here’s exactly how it went down.
Opening the Notebook & First Approach
Grabbed my chemistry notes first. Recalled the formula involves carbon, hydrogen, nitrogen, oxygen, and phosphorus. Started jotting symbols: C, H, N, O, P. Easy peasy so far, right? Then I naively counted atoms straight from memory – 10 carbons, a bunch of hydrogens, some nitrogens… slapped numbers together like building blocks.
First big oops moment: I wrote C10H12N5O13P3. Felt smug until I thought, “Wait, does that even add up mass-wise?”
Pitfall Parade: Charge Confusion & Grouping Goofs
Then I remembered ATP has negative charges. Panicked a little. Added “-4” at the end like an afterthought. Totally forgot that charges aren’t just stickers you slap on formulas – they actually change how atoms connect. Also, lazily treated the whole thing as one chunk instead of breaking it into adenosine + triphosphate parts. Messy.
Key mistakes stacked up fast:
- Ignored how phosphates link to each other & ribose
- Forgot hydrogen adjustments where bonds form
- Mixed up oxygen atoms between the rings and phosphate chains
Frustration Mode & Fixing the Mess
Scratched my head, flipped textbooks open. Decided to rebuild step-by-step:
- Adenosine part: Drew the adenine and ribose rings properly – C10H12N5O4
- Phosphate chain: Visualized three PO4 groups linking up – that’s H4P3O10 before connecting
- Combine them: Linked the ribose to first phosphate, which meant removing H from ribose and H from phosphate. Poof! Atoms adjusted.
Lightbulb moment: Those hydrogens? When bonds form, they disappear from the final count. My original numbers were inflated because I double-counted bond spots.
The Correct Path Forward
Finally nailed it: C10H16N5O13P3 for the whole structure, but crucially remembered the -4 charge matters for real chemical behavior. Double-checked mass balance too – phosphates weren’t floating islands anymore.
Biggest takeaways from this facepalm session:
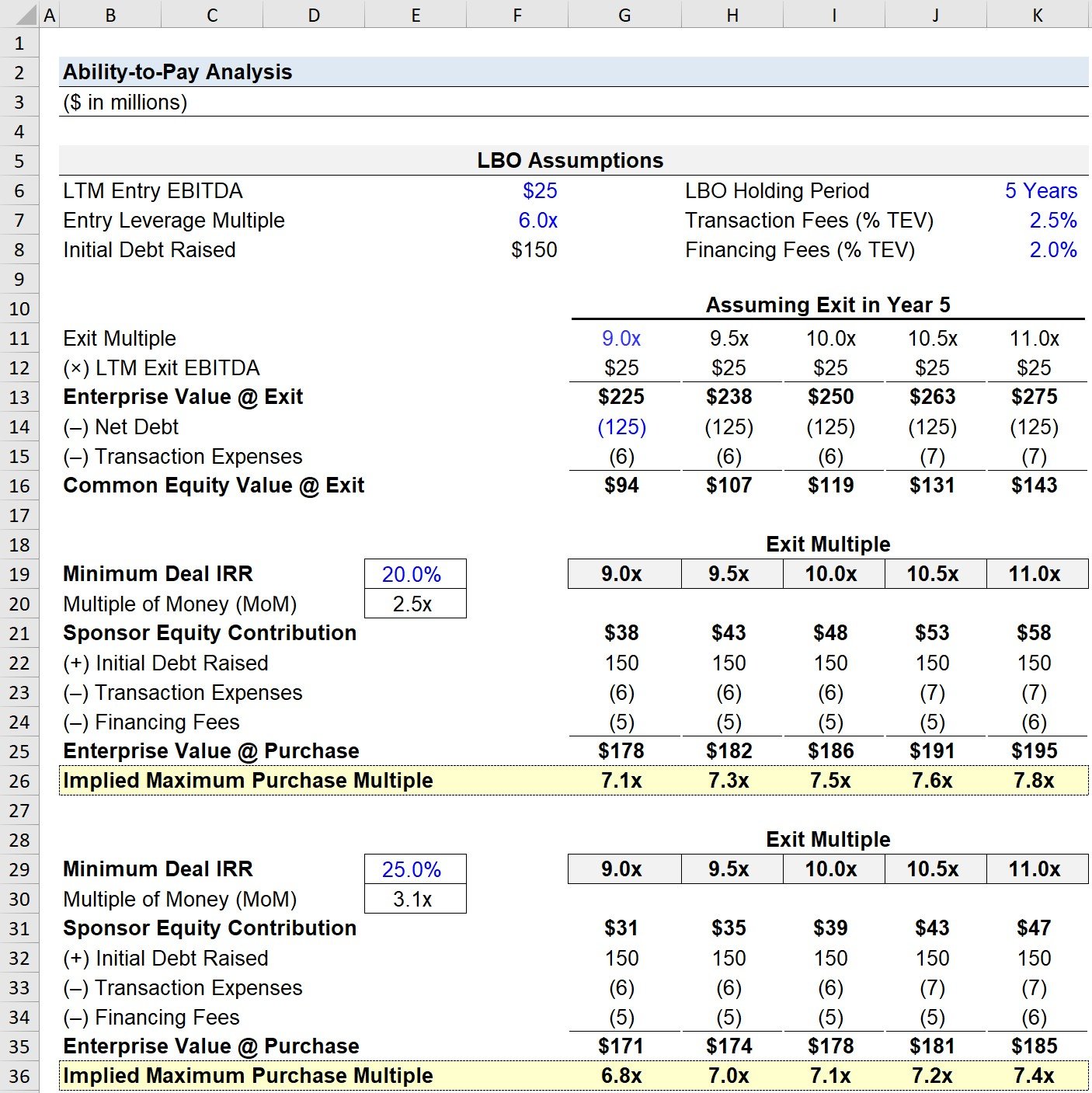
- Never trust atom memory – always sketch components separately
- Hydrogens vanish where bonds form (stop overcounting!)
- Charges aren’t optional decorations
- Phosphate chains need structural respect, not lazy sums
Honestly? Feels good to relearn basics wrong so publicly. Hopefully saves someone else an hour of frustrated scribbling!
